- cross-posted to:
- health@lemmy.world
- cross-posted to:
- health@lemmy.world
The Food and Drug Administration may be one step closer to what could be the first approval of a drug that uses the groundbreaking gene-editing tool CRISPR.
The drug, called exa-cel, treats sickle cell disease, an inherited blood disorder that affects an estimated 100,000 people in the U.S., most of whom are Black, according to the Centers for Disease Control and Prevention.
The illness causes the body’s red blood cells, usually disk-shaped, to take on a crescent or sickle shape. When that occurs, the cells can clump together, leading to clots and blockages in the blood vessels. That may result in a variety of complications, including excruciating pain, trouble breathing or stroke.


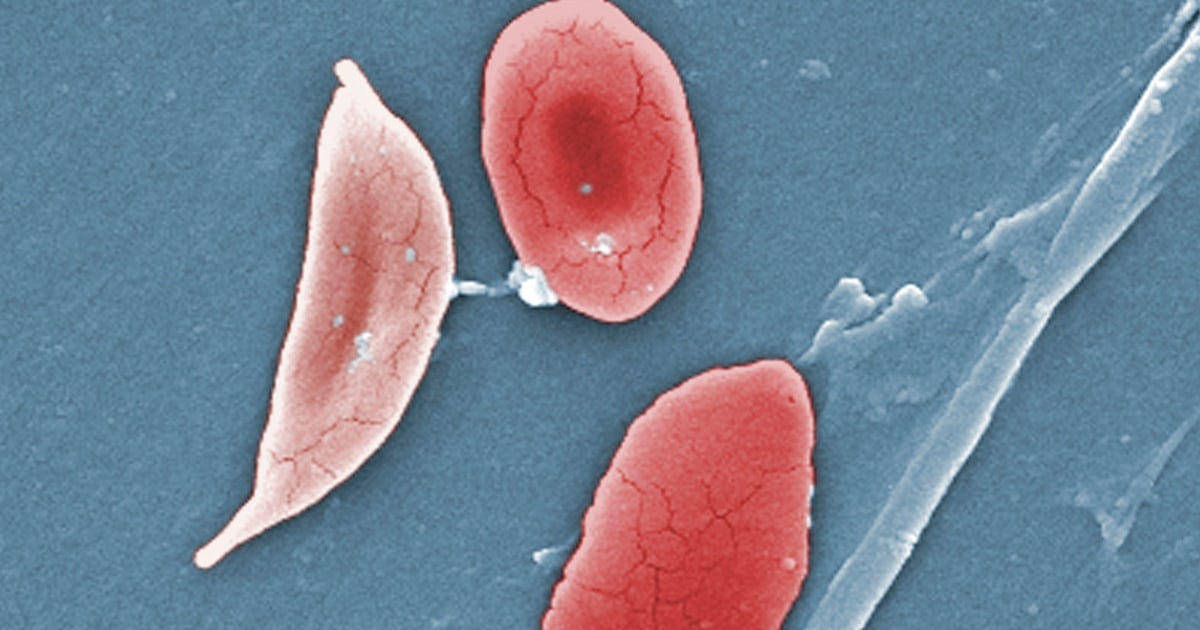
Oddly enough those with the sickle cell trait (one abnormal gene and one regular gene) actually have protection against malaria. Those with sickle cell anemia (2 abnormal genes) unfortunately, do not.
Oh, it’s more complicated than I thought.