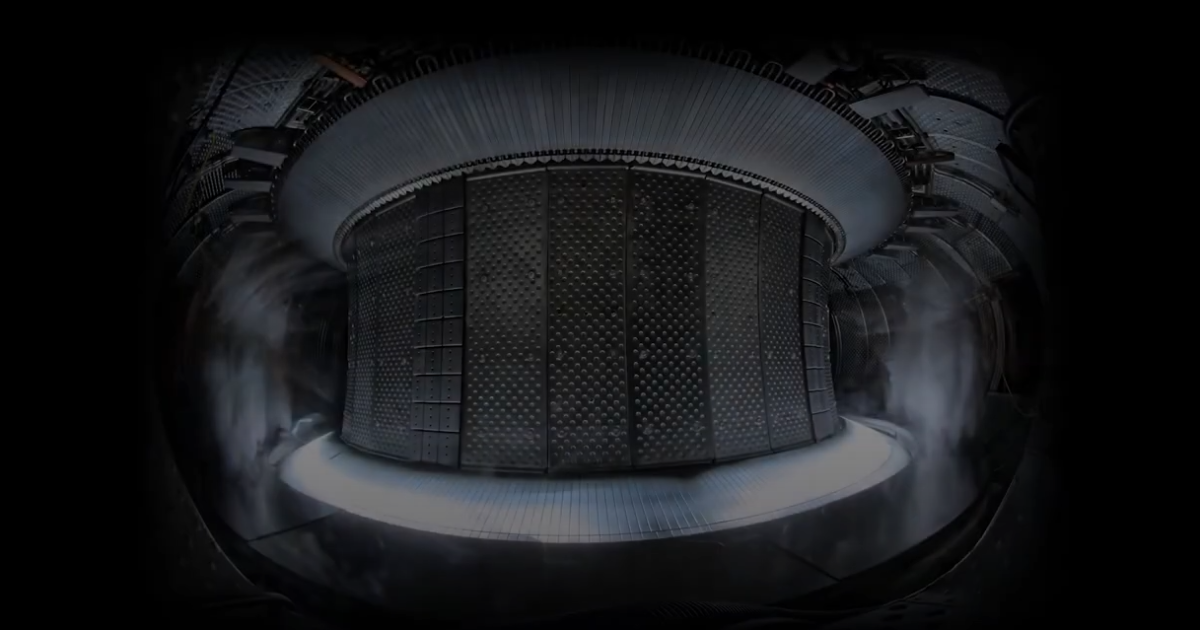
France has upped the ante in the quest for fusion power by maintaining a plasma reaction for over 22 minutes – a new record.
In the latest test, the WEST Tokamak held its reaction for 1,337 seconds.
That’s not just badass, that’s elite.
Achieving the dream of commercial fusion power is the Holy Grail of engineering and has been for 80 years. With a single gram of hydrogen isotopes yielding the energy equivalent of 11 tonnes of coal, a practical fusion reactor would hold the promise of unlimited, clean energy for humanity until the end of time.
Do these fusion reactors require “heavy” hydrogen?
If so, does that affect how usable they are / what can serve as “fuel?”
Look I’m way way long in the tooth since I took nuclear chemistry, and even then it was a cursory training, but afaik, I think modern fusion reactors need an amount of hydrogen to get started, but then, effectively, they are ‘breeding’ either deuterium or tritium in situ, from the walls of the reactor.
Even if they didn’t, a gram is such a phenomenally small amount when most of the universe is hydrogen.
I’m not knowledgeable enough to give a definite answer to this and dont wanna give an answer that may be wrong. But thats a good question
The fusion reaction with the lowest requirements is the deuterium + tritium reaction. Both are heavy hydrogen isotopes. A common hydrogen nucleus consists of a single proton, a deuterium nucleus consists of a proton and neutron, and a tritium nucleus consists of a proton and two neutrons. The first two are stable while the last is radioactive.
Deuterium is rare but naturally present on Earth. It’s no challenge to harvest it from water. You can come across some fun facts when you talk to fusion folks. The top inch of San Diego Bay has enough deuterium to power the city for a year, when fused with tritium in a “burning plasma”.
Tritium is not naturally present in harvestable quantities on Earth. This has to be bred through other reactions. The reaction of interest is lithium reacting with a neutron (a neutron is a product of the fusion reaction) which produces tritium and other products.
Effectively, the easiest fusion reaction involves “burning” deuterium and lithium.
Now, there are other fusion reactions that don’t involve tritium, but they are more challenging physically, and some have their own challenges for sourcing the required fuel. Almost all fusion reactor proposals today are based on the deuterium + tritium fusion reaction.
Ran for 1337 seconds 😎
The tricky bit isn’t to get atoms to fuse. That’s a fairly simple lab bench experiment. The problem is creating the right conditions where the fusion reaction is self-sustaining, with a net energy output. That means reaching temperatures of between 100 – 150 million °C (180 – 270 million °F, or 3-5 times hotter than the Sun’s core), a pressure of five to 10 atmospheres at the point of reaction, and keeping a high-energy plasma stable for at least 10 seconds.
Keep a stable reaction while pulling energy from it (and pull enough energy so it doesn’t overheat and collapse the superconducting magnetic coils but not so much energy that the fusion stalls)